Diffuse Intrinsic Pontine Glioma and Diffuse Midline Glioma
Brain tumors are the second most common cancer entity after leukemia. In Germany, around 30 children are diagnosed with a particularly malignant brain tumor every year: diffuse midline glioma (DMG), including diffuse intrinsic pontine glioma (DIPG), represent the most aggressive brain tumor entity with very poor prognosis and overall-survival rates of less than 2 % due to ineffective therapeutic options. This miserable situation has not been changed for decades. In 2012, mutations in histone 3 gene variants (H3K27M) had been discovered to be present in 85% of all DIPG cases which probably contribute significantly to their aggressiveness. Therefore, understanding the effects caused by H3K27M-mutation could be the basis for discovering new effective therapeutic approaches.
Overview
The majority of all DIPG are characterized by mutation in a histone 3 gene variant (mutation: H3K27M), resulting in global chromatin changes and even worse survival. Affected patients show almost no prospect of cure. In consequence, targeting H3K27M-mutation in DIPG/DMG might be a promising therapeutic approach. To characterize the specific effects of H3K27M on (epi)genetic level and tumor biology as well as to identify H3K27M-specific therapeutic targets, we generated H3K27M-specific isogenic tumor models from DIPG/DMG cells, either originally carrying H3K27M or histone 3 wildtype (H3WT). We found that H3K27M-mutation is indeed essential for H3.3K27M-mutated DIPG/DMG cell growth and proliferation, migration/invasion, and stemness potential associated with radiochemotherapy resistance. These findings indicate the essential contribution of H3.3K27M to the poor survival of DIPG/DMG patients. In consequence, targeting H3K27M-dependent factors supporting the malignant tumor phenotype of DMG/DIPG might be a promising therapeutic approach to improve treatment of affected children.
Research Focus
Investigation of epigenetic key players in dependence of H3-mutation status in DIPG/DMG
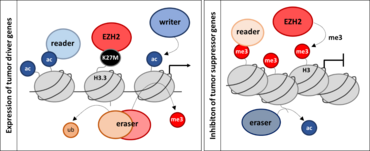
Mutation in H3K27 results in increased malignant phenotype accompanied by a stronger impact on tumor characteristics such as proliferation, migration and invasion as well as stemness potential associated with therapy resistance. We aim to investigate the particular role of contributing key players which function might be changed dependent on H3-mutation status. To this end, the biological effects and epi(genetic) impact of different writers, readers and erasers are analyzed in isogenic DIPG/DMG cell lines with and without H3K27M-mutation. Understanding the (epi)genetic and consequential biological special features of DIPG/DMG might be the first step towards effective therapeutic approaches.
Publications
Cooperations
Marta M. Alonso, PhD, Dpt. of Pediatrics/Solid Tumors Program, Clínica Universidad de Navarra-CIMA
Dr. Angel M. Carcaboso, Pediatric Hematology and Oncology, Hospital Sant Joan de Deu / Institut de Recerca Sant Joan de Deu, Barcelona, Spain
PD Dr. Christian Dullin, Department of Diagnostic Radiology/Translational Molecular Imaging, University Medical Center Göttingen, Germany
PD Dr. Niklas Engels, Institute for Cellular & Molecular Immunology, University Medical Center Göttingen, Goettingen, Germany
Dr. Esther Hulleman, Pediatric Neuro Oncology Research, Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands
Prof. Steven A. Johnsen, Robert Bosch Center for Tumor Diseases, Stuttgart, Germany
Prof. Dr. Martin S. Staege/Kristina Engel, Klinik für Pädiatrie, Universitätsklinikum Halle, Germany
Dr. Sven Schuchardt, Fraunhofer-Institut für Toxikologie und Experimentelle Medizin ITEM, Hannover, Germany
Dr. Maria-Patapia Zafeiriou, Department of Pharmacology and Toxicology, University Medical Center Göttingen, Germany
Funding
Deutsche Forschungsgemeinschaft
Deutsche Kinderkrebsstiftung
The Cure Starts Now (https://thecurestartsnow.org/research/research-and-grants/university-medicine-goettingen-2023-05-19/)
Menschen für Kinder e.V. Albshausen
Kubeschka/Stricker/Wirth-Stiftung Stiftung der Georg-August-Universität
Heidenreich von Siebold Programm Universitätsmedizin Göttingen
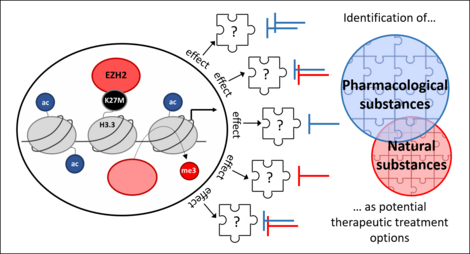
Identification of therapeutic treatment strategies for DIPG/DMG
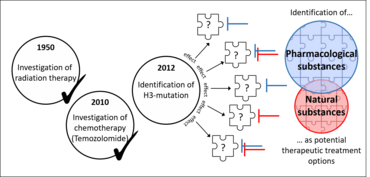
Comparison of H3K27M-mutated and H3WT DIPG/DMG cells revealed strong differences between histone-mutated DIPG/DMG cells and non-histone-mutated ones with regards to e.g. their phenotype, epigenetic landscape or transcriptome.
To improve the clinical outcome for affected children, we aim to discover potential therapeutic target molecules and second to investigate a therapeutic strategy based on our knowledge gained by comparing of H3K27M-mutated and non-mutated isogenic cell lines. In addition, we focus on therapeutic options that might be beneficial for all DIPG/DMG patients, independent of their H3-mutation status.
To this end, we are also interested in combination of drugs with current standard radiochemotherapy in order to find potential therapies that might increase the response of tumor cells to standard treatment and decrease the aggressive potential of H3K27M-mutated and non-mutated DIPG/DMG.
Publications
Cooperations
Marta M. Alonso, PhD, Dpt. of Pediatrics/Solid Tumors Program, Clínica Universidad de Navarra-CIMA
Dr. Angel M. Carcaboso, Pediatric Hematology and Oncology, Hospital Sant Joan de Deu / Institut de Recerca Sant Joan de Deu, Barcelona, Spain
PD Dr. Christian Dullin, Department of Diagnostic Radiology/Translational Molecular Imaging, University Medical Center Göttingen, Germany
PD Dr. Niklas Engels, Institute for Cellular & Molecular Immunology, University Medical Center Göttingen, Goettingen, Germany
Dr. Esther Hulleman, Pediatric Neuro Oncology Research, Princess Máxima Center for Pediatric Oncology, Utrecht, The Netherlands
Prof. Steven A. Johnsen, Robert Bosch Center for Tumor Diseases, Stuttgart, Germany
Prof. Dr. Martin S. Staege/Kristina Engel, Klinik für Pädiatrie, Universitätsklinikum Halle, Germany
Dr. Sven Schuchardt, Fraunhofer-Institut für Toxikologie und Experimentelle Medizin ITEM, Hannover, Germany
Dr. Maria-Patapia Zafeiriou, Department of Pharmacology and Toxicology, University Medical Center Göttingen, Germany
Funding
Deutsche Forschungsgemeinschaft
Deutsche Kinderkrebsstiftung
The Cure Starts Now (https://thecurestartsnow.org/research/research-and-grants/university-medicine-goettingen-2023-05-19/)
Menschen für Kinder e.V. Albshausen
Kubeschka/Stricker/Wirth-Stiftung Stiftung der Georg-August-Universität
Heidenreich von Siebold Programm Universitätsmedizin Göttingen
Research Team

Principal Investigators
Prof. Dr. med. Christof Kramm

Medical Director of the Department of Pediatric Hematology and Oncology
Contact: christof.kramm(at)med.uni-goettingen.de
Dr. rer. nat. Maria Wiese
Maria Wiese studied Biology at the University of Göttingen which she completed with a diploma in 2008. She conducted her PhD thesis in the Department of Hematology and Oncology at the University Medical Center Göttingen studying the function of B9L transcription factor and its role for canonical Wnt-signaling in colon cancer which she defended in 2012 with summa cum laude. After her PhD she continued her work on B9L-mediated transcriptional regulation in colon cancer as a postdoc. In 2015 she joined the research group of Prof. Dr. med. Christof Kramm as postdoc exploring signaling pathways in pediatric high grade glioma. During her studies she became passionate about investigating the mechanisms of transcriptional epigenetic regulation and became group leader of the Experimental Pediatric Neurooncology (Division of Pediatric Hematology and Oncology) at the University Medical Center Göttingen.
Contact: maria.wiese(at)med.uni-goettingen.de
Postdoctoral Fellows
PhD/MD Students
M.Sc. Effrosyni Kapsali (PhD thesis)
Maren Hambach (MD thesis)
Jonas Ruben Vetter (MD thesis)
Alexa Julia Katharina Meyer Vandehult (MD thesis)
Elisabeth Longmei Michels (MD thesis)
Tobias Lüken (MD thesis)
Stefanie Sander (MD thesis)